NOTES
ON
THE USE OF
A N T H R A C I T E
IN
THE
MANUFACTURE
OF IRON.
WITH
SOME REMARKS
ON
ITS
EVAPORATING
POWER.
BY
WALTER R. JOHNSON, A. M.,
CIVIL
AND MINING ENGINEER; PROFESSOR OF CHEMISTRY AND NATURAL PHI-
LOSOPHY
IN THE MEDICAL DEPARTMENT OF PENNSYLVANIA COLLEGE; LATE
PROFESSOR
OF MECHANICS AND NATURAL PHILOSOPHY IN THE FRANK-
LIN
INSTITUTE, PHILADELPHIA; MEMBER OF THE NATIONAL IN-
STITUTION
FOR THE PROMOTION OF SCIENCE; OF THE ACAD-
EMY
OF NATURAL SCIENCE OF PHILADELPHIA; OF THE
ASSOCIATION
OF AMERICAN GEOLOGISTS, &C. &C.
BOSTON:
___________
CHARLES C.
LITTLE AND JAMES BROWN.
________
MDCCCXLI.
Pages 28 – 101.
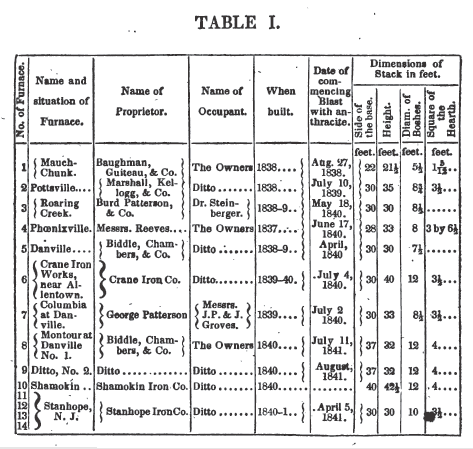
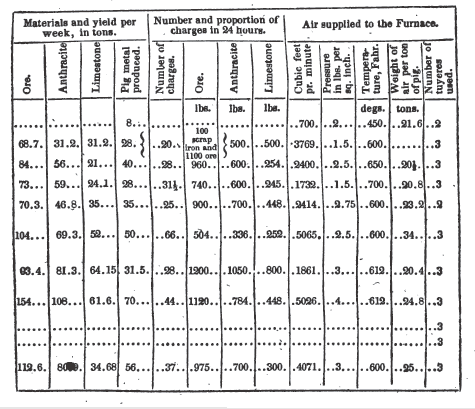
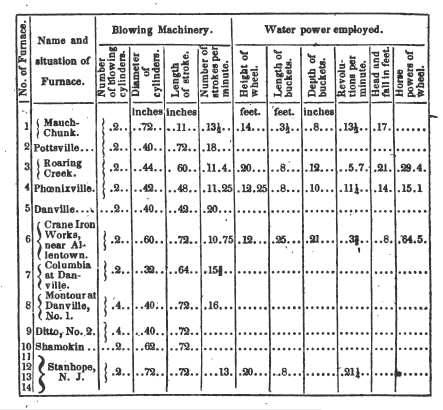
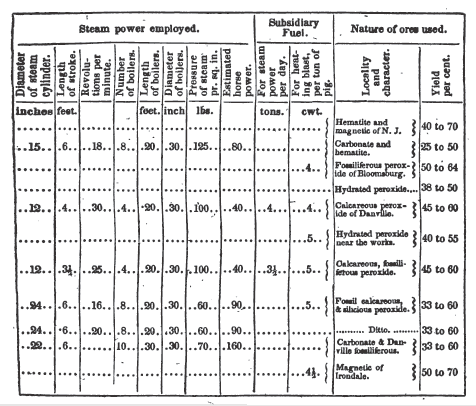
The following is a tabular view
of the blast furnaces, applied to the manufacture of iron with the anthracite
of Pennsylvania.
Remarks on the preceding Table.
1. MAUCH CHUNK FURNACE.
The
furnace at Mauch Chunk, which stands at the head of the preceding table, is
believed to have been the first in this country, at which any considerable
success was attained in the smelting of iron with anthracite.* The iron produced was of various, but
mostly inferior qualities, owing probably to a deficiency of blast. The blowing
cylinders are of wood, (single acting) and at the speed employed did not
furnish over 700 cubic feet of air per minute. Their apparatus for hot blast
was at first defective, and was afterwards placed at the trunnel head, where it
could not be so well regulated as if arranged in separate ovens, with an
independent fire. Hence, even of the limited supply of air taken into the
bellows, a considerable portion must have been lost by leakage, and by escapes
at the open tuyeres then applied.
* Mr. Crane's patent in this country, bears date November 29, 1838. The
Mauch Chunk furnace went into blast for the second time, about the same day. The operations at Pottsville
were commenced July 10, 1839. But the trial of three months' continuance, began
about the 20th of October, 1839, and its completion was celebrated on the 18th
of January, 1840. The blast of 100 days, terminated at Mauch Chunk, November 2d,
1839. The following letter shows the period at which Messrs. Baughman, Guiteau
& Co. commenced their works— with other particulars worthy of notice.
Beaver Meadow, November
9, 1840.
SIR,
Agreeable to a request of Col. Henry High, of Reading,
I send you the following hastily written statement of the experiments made by
Baughman, Guiteau & Co., in the smelting of iron ore, with anthracite coal
as a fuel.
During the fall and winter of the year 1837, Messrs. Joseph
Baughman, Julius Guiteau, and Henry High, of Reading, made their first
experiment in smelting iron ore with anthracite coal, in an old furnace at
Mauch Chunk, temporarily fitted up for the purpose; they used about eighty per
cent of anthracite, and the result was such as to surprise those who witnessed
it (for it was considered as an impossibility even by iron masters); and to
encourage the persons engaged in it, to go on. In order, therefore, to test the
matter more thoroughly, they built a furnace on a small scale, near the Mauch
Chunk Weigh Lock, which was completed during the month of July, 1838.
Dimensions.
Stack, 21 1-2 feet high, 22 feet square at the base.
Boshes, 5 1-2 feet across.
Hearth, 14 by 16 inches in the square, and 4 feet 9
inches from the dam stone to the back.
Blowing apparatus consisted of two cylinders, each 6
feet diameter; a receiver, same diameter, and about 2 1-2 feet deep; stroke, 11
inches. Each piston making from 12 to 15 strokes per minute.
An overshot water-wheel, diameter 14 feet; length of
bucket 3 1-2 feet; number of buckets, 36; revolutions per minute, from 12 to
15.
The blast was applied August 27th, and the furnace
kept in blast until September 10th, when they were obliged to stop in
consequence of the apparatus for heating the blast proving to be too temporary.
Several tons of iron were produced of Nos. 2 and 3 quality. I do not recollect
the proportion of anthracite used. Temperature of the blast did not exceed 200¡
Fahrenheit.
A new and good apparatus for heating the blast was
next procured, (it was at this time I became a partner in the firm of B., G.
& Co.) consisting of 200 feet in length, of cast iron pipes, 1-1/2 inches
thick; it was placed in a brick chamber, at the trunnel head, and heated by a
flame issuing thence.
The blast was again applied about the last of
November, 1838, and the furnace worked remarkably well for five weeks,
exclusively with anthracite coal; we were obliged, however, for want of ore, to
blow out on the 12th of January, 1839. During this experiment, our doors were
open to the public, and we were watched very closely both day and night, for
men could hardly believe what they saw with their own eyes, so incredulous was
the public in regard to the matter at that time; some iron masters expressed
themselves astonished, that a furnace could work whilst using unburnt, unwashed, frozen ore, such as
was put into our furnace.
The amount of iron produced, was about 1 1-2 tons per
day, when working best, of Nos. l, 2, and 3 quality.
The average temperature of the blast was 400¡
Fahrenheit.
The following season we enlarged the hearth to 19 by
21 inches, and 5 feet 3 inches from the dam stone to the back of hearth; and on
July 26th, the furnace was again put in blast, and continued in blast until
November 2d, 1839, a few days after the dissolution of our firm, when it was
blown out in good order. For about three months we used no other fuel than
anthracite, and produced about 100 tons of iron, of good Nos. 1, 2, and 3
quality. When working best, the furnace produced about 2 tons per day.
Temperature of the blast was from 400¡ to 600¡ Fahrenheit. The following ores
were used by us, viz. "Pipe ore," from Miller's mine, a few miles
from Allentown; "brown hematite," commonly called top mine, or
surface ore; "rock ore," from Dickerson's mine in New Jersey; and
"Williams township ore," in Northampton county. The last mentioned
ore produced a very strong iron, and most beautiful cinder.
The above experiments were prosecuted under the most
discouraging circumstances, and if we gain any thing by it, it can only be the
credit of acting the part of pioneers in a praiseworthy undertaking.
Most respectfully, Sir,
Your obedient servant;
F. C. LOWTHORP.
[Ed.
The Mauch Chunk Courier carried
articles about the failure and dissolution of
this furnace in November, 1839. J.McV]
PROF. WALTER R. JOHNSON, Philadelphia.
It is proper to mention that the first account which
reached this country relative to the operations of Mr. Crane, in manufacturing
iron with anthracite in Wales, was in the proceedings of the Liverpool meeting
of the British Association, for the year 1837. That meeting was held in
September of that year, and the statement contained in the 6th volume of the
proceedings of that body, page 52, (transactions of sections) is, that his
operations were commenced with hot air, on the 7th of Feb., 1837. The following
extracts are perhaps sufficient to convey a knowledge of the most important
results then obtained.
" One of the three furnaces at present on the
establishment, is a small cupola furnace, built from the top of the hearth with
fire bricks only. This cupola is of the following dimensions; 41 feet in its
whole height, 10 1-2 feet across the boshes, and the walls of the thickness of
two nine-inch bricks; the hearth 3 feet 6 inches square and 5 feet deep."
"I have produced, from the cupola furnace, the ton of iron in the smelting
process, on the average of three months, with less than 27 cwt. of anthracite
coal; the heating of the blast and the calcination of the mine, require, of
course, upon my plan, the same quantity of fuel which is necessary for the like
processes in other establishments." "Since I have adopted the use of
anthracite coal, combined with hot air, the produce of the furnace, with a
pressure of 1 1-4 pounds per square inch, has ranged from 30 to 34 and 36
tons." "Its present weekly average may be expected to range from 35
to 36 tons." " With respect to the quality of the iron produced by
the combination of hot blast and anthracite, the result is very satisfactory.
It is well known that my cold blast iron, for all purposes where great strength
was required, was never deemed inferior to any smelted in South Wales. That
which I have hitherto produced with hot blast and anthracite coal, is, however,
decidedly stronger than any other before smelted at the Ynescedwin iron
works."
2. POTTSVILLE FURNACE.
The
Pottsville furnace is the same with which Mr. William Lyman made his
experiments, which commenced at the date mentioned in the table. The continuous
blast of three months, required by the conditions under which he received this
furnace property, was completed in January, 1840. Since that period several
occurrences have conspired to disturb the regularity of action in this
establishment. At one time an attempt was made to heat the air in close
furnaces, throwing it, in part, through, and in part over, the
fire, which heated the blast, and thus sending in the gaseous products of
combustion, as well as atmospheric
air, to supply the furnace. This attempt failed, either because carbonic acid,
nitrogen and sulphurous acid gas interfered too much with the combustion of the
oxygen in that portion of air which had escaped the action of the heating fire,
or because the cases or furnaces in which the air heating fires were contained,
proved inadequate to sustain the pressure, and thus supplied an insufficient
quantity of the mixture to give a vigorous and powerful reducing heat at a
proper height above the hearth.
This apparatus was used but for a short time, and when blown out, to re-establish the semicircular-tube system, the hearth was found greatly enlarged, as might have been inferred from the black, heavy, though porous cinder which was the only kind obtained during the time this apparatus was in use. In fact, it appears that the unreduced oxide of iron came down and served as a flux to the hearth stone; a result which I have often known to occur where a deficient blast allowed any considerable portion of the ore to escape reduction. Pure pig metal, when fairly collected in the hearth, has no action on good fire-stone or fire-brick, any more than has. the well-reduced vitreous cinder. which ought to accompany the production of good foundry iron.
Besides the difficulties attending the air heating apparatus, which has again given way, the Pottsville furnace his been supplied with ores of almost every variety, mixed, or used separately without proper discrimination, and sometimes, it is alleged, the stock has become, nearly or quite exhausted, leaving the works to go on without any addition of ore for hour together. It is not surprising that, under these circumstances, iron of very different qualities should be produced, and that this furnace should, with all its advantages of being situated amidst the greatest abundance of anthracite, be able to render a less satisfactory result of the anthracite iron manufacture than those which have fewer apparent advantages.*
*A second furnace near Pottsville, called the Valley Furnace, was put into blast September 17,1841, and is represented to have succeeded admirably from the first moment of its action. It uses only the ore found upon the ground in connection with the anthracite beds.
The iron, recently made at Pottsville has been cast from a cupola either into T rails, for mine roads, or into cannon balls for the government. That it is of good quality for the former purpose I had an opportunity, through the kindness of the. proprietor, of testing, by actual experiment. A rail was taken at random from a large pile, recently cast, and subjected to the following test:
The rail was 6 feet long, and had the form of cross section, represented in the annexed figure, containing in its area 3.1 square inches. It had at each end, for about 3 inches, along. the base, wings or flanges for securing it to the cross-ties, as represented by the dotted lines X and Y, and in the middle of its length similar wings, 6 inches long, or 3 inches on each side of the centre. The rail weighed 66 lbs., or 33 lbs. per yard. To prove its flexibility and strength, its two extremities were placed on supports 5 feet 9 inches apart and of such height as to allow of the suspension of weights beneath the centre of the bar. A strong stirrup was placed over the rail, before placing the latter on its supports, and carried to the centre. To this chains, supporting the weights were attached; A straight-edged, broad ruler was adjusted beneath the rail, from which the deflections could be easily measured.
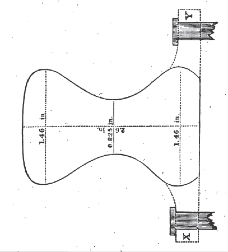
The following weights were then added and observations made:
1. With 320 lbs. (including, of course, the chains and stirrup) a deflection of 0.02 inch.
2. With 1040 lbs. a deflection of 0.20 inch.
3. With 2000 lbs. a deflection of 0.37 inch.
4. With 2525 lbs. a deflection of 0.50 inch.
5. With 3000 lbs. broke.
This last weight was sustained about 4 minutes before the rail gave way.
The fracture took place just outside of the wing already mentioned, and, of course, 3 inches from the centre. Hence the strengthening effect of the wing was proved, and led me to recommend a pattern, in which the wings should extend the whole length of the rail.
Another rail, intended to sustain locomotives, made of the same iron, and on a pattern entirely similar to the first, was also partially tested, but not broken.
It had the following dimensions, viz.
Length, 6 feet
Depth, 4 inches;
Breadth at top, 2.5 inches
Breadth at bottom, 2.5 inches
Breadth of mid-rib, 1.25 inches
Weight, 134 lbs. or 67 lbs. per yard; consequently its area of cross section was almost exactly double that of the first rail.
This rail having been placed on the two supports, at the same distance apart as in the first trial, weights were applied and deflections observed as follows, viz.
1000 lbs. produced a deflection of 0.08 inch.
2000 lbs. produced a deflection of 0.15 inch.
3000 lbs. produced a deflection of 0.20 inch.
4000 lbs. produced a deflection of 0.26 inch.
4500 lbs. produced a deflection of 0.28 inch.
After this weight had been applied, the arrangement of props to preserve the - supports erect gave way, and time not allowing a repetition of the trials, they were given over for the present, having, as was believed, satisfactorily proved that a rail made of this iron, of the dimensions above tested, would, when supported both at the ends and centre, be adequate to sustain the weight of any locomotive now in use. The, strength, when supported in the middle as well as at the ends, being double - of what. it is when supported at the ends alone, if we take the strength of the first rail as a standard, and compare the breadths and squares of the depths of the two cross sections together, we shall find, that, so far from having reached the ultimate strength of the large rail, with a weight of 4500 lbs. on a length of 69 inches, the latter would have required 12,158 lbs. to break it. Placed on cross ties 3 feet apart, the larger rail ought to bear about 12 tons as its ultimate load.
3.
ROARING CREEK FURNACE
The Roaring Creek Furnace stands about one fourth of a mile up the creek, above its mouth which is in the north branch of the Susquehanna, three miles below the town of Catawissa, and five miles above Danville.
The reason of selecting this position, was in order to take advantage of the valuable water power of this stream, which, in the course of a mile or a little more, has a fall of not less than fifty feet. It is, however, not altogether free from objection, on account of the occasional failure of water in dry seasons.
The ore is the rich fossiliferous kind, from the neighborhood of Bloomsburg, distant about six or seven miles and the limestone is also brought from the north side of the river, a distance of two or three miles. The coal is from Wilkesbarre, distant about forty miles, by the line of the North, Branch canal. The water wheel appears to me to fulfill its purpose but imperfectly, and the machinery to move with considerable irregularity, owing in part to the want of counterpoises to the cranks and connecting rods of the blowing cylinders, which are laid horizontally; thus adding half the weight of the two long connecting rods and that of the two heavy cast iron cranks, to the regular resistance of the air in the cylinders, and by so much increasing for the moment the quantity of work to be done by the water wheel; and on the opposite part of the revolutions, the contrary effect takes place, to an extent which becomes very sensible in the movements of machinery, as well as in the intensity of the blast. The heating of 'the blast is effected on the plan of the Calder works.
The volume and pressure of air for this furnace, given in the table, was derived from the statement of the occupant, as that used in ordinary times; but at the period of my visit, September 12, 1840, the lowness of the stream caused a considerable reduction of volume, being then only 1672 cubic feet per minute, under a pressure of 1.382 pounds per square inch. The yield was then but 35 tons per week, and diminishing.
The pig metal made at the Roaring Creek Furnace is of excellent quality, being generally grey No. 1, and exceedingly well suited to foundry purposes. It has also been fully proved in regard to its adaptation to the purposes of making bar iron, by the Messrs. Whitaker, at Reading, who have, it is said, offered strong testimony in its favor,
By urging the furnace to its utmost with burthen, there was obtained for a few days a yield equal to 72 tons - per week; but the metal was, of course, inferior in quality to the ordinary product.
The cost of this establishment, independent of the site, was $31,000.
4.
PHOENIX FURNACE.
The furnace at Phoenixville, situated twenty. five miles from Philadelphia, directly on the line of the Schuylkill navigation, is supplied with anthracite from Pottsville, and with ore from Yellow Springs, which contains a large portion of silica. The pig metal is grey No. 2, moderately soft, but wants toughness. Bar iron, manufactured from the pig of Phoenixville, is generally cold short. The burning out of hot air pipes and the destruction of hearthstones, consequent, as is believed, on- a deficient blast, have been frequent causes of embarrassment at these works. The ore yields 38.3 per cent., and about 134 tons of coal are required to make one ton of pig metal. The cost of building Phoenixville furnace, independent of wheel house and dwellings, was $7949. This includes cast and bridge-houses.
5.
DANVILLE FURNACE.
The Danville works use anthracite, from Wilkesbarre, received by the North Branch canal, and ore obtained within half a mile of the furnace. Two or three varieties of the latter are found within a short distance of each other. The calcereous fossiliferous ore of Montour's ridge, yielding from 56 to 64 per cent of metallic iron, is the chief reliance of the works; but large portions of the hard siliceous band ore, mined immediately in the neighborhood, is also extensively used. Both the soft and hard. beds probably underlie the site of the works. The pig metal is of a dark grey color, granular texture, soft and fusible, well adapted for foundry purposes; and represented to be in no respect inferior to the best Scotch pig. The same remarks will apply to the products of furnaces Nos. 7 and 8.
The
pressure of blast in this furnace is measured and regulated by a safety valve,
loaded directly with weights to the amount of 2 pounds per square inch, and
under this load the air constantly escaped in moderate quantity at the time of
my visit.
6. CRANE FURNACE.—NO. 1.
The
Crane Iron works have been erected under the immediate direction of Mr. David
Thomas, who had been previously engaged at the establishment of Mr. Crane, in
Wales. They are situated about three miles from Allentown, on the line of the
Lehigh navigation. The volume of air passing through the bellows, is the
quantity given in the table, computed from the known length of stroke and
diameter of piston, together with the observed number of strokes per minute,
and it is this volume, assumed to be under the pressure also noted in the
table, which I was assured by Mr. Thomas, was the load on the safety valve when
examining the works. A considerable quantity of air was escaping at the safety
valve, and a part is used to supply the heating ovens for hot blast, as is also
done at Roaring Creek and elsewhere. Of the economy of this latter arrangement,
except where very fine coal is used for heating, I am disposed strongly to
doubt. A single high chimney, with suitable register, to regulate the draught,
might, I apprehend, be entirely equivalent, and being self-acting, would
require no constant expense of power to maintain the combustion. The water
wheel was intended to supply two furnaces.
The
stock at this furnace is very
expeditiously elevated from the level of the base of the stack, by means of
water pumped up by the blast wheel, into a cistern near the trunnel head, and
which is thence allowed to flow alternately into two boxes of suitable dimensions,
suspended by a chain passing over a pulley in such a manner, that the descent
of one box filled with water, and bearing on its cover the empty barrows for
stock, elevates the other box now emptied of water, but carrying up the
barrows, loaded with ore, coal, and limestone.
The
blast in this establishment is heated in four ovens, each having twelve arched
tubes of five inches interior diameter, and two inches thickness of cast iron.
The temperature, when tried in my presence, was not sufficient to melt lead,
though it was understood to be, in general, capable of producing that effect.
The
ore chiefly used at this furnace, is the hematite or hydrated peroxide of iron,
of which about 2-1/2 tons are required to make 1 ton of pig metal. It is used entirely
in its raw state. It was stated to cost at the works $2.25 per ton. The
anthracite is from the mines of the Lehigh Coal Company, near Mauch Chunk, of
which 87.5 per cent is carbon, and 5.5 earthy matter. A quantity of the metal
made at this furnace has been puddled with anthracite, at Boonton, New Jersey,
and produced excellent fibrous bar iron. It was stated, that 21 cwt. of pig
produced 20 cwt. of puddled iron, thus showing a loss in the first process of
making bar iron of only 4.76 per cent and that 22 1-2 cwt. made a ton of bars,
showing altogether a loss of but 11.11 per cent. The foundation of a second
furnace is prepared.
7. COLUMBIA FURNACE.
At the Columbia Furnace, in Danville, an attempt was at first made to heat the blast in a chamber above the trunnel head, but the pipes were soon burned away, and leaked to such a degree as to lose a large portion of the blast. The engine was, at the same time, too small and deficient in power. A succession of nozzles of different sizes, viz., 1-1/4, 1-1/2, 1-3/4, 2, and 2-1/2 inches in diameter, was tried. The greatest yield of iron during the first blast, which lasted only five weeks, was 5 tons in 21 hours, and during that time the 2-inch nozzle was employed. When, after this blast, the furnace was blown out, the hearth and inwalls were found very much cut away, the former being enlarged from 3-1/2 to 6 feet in diameter. The cinder, as usually happens when similar destruction is going on, was constantly black, and often highly porous.
The most successful operations were performed while the blast was most powerful, the cinder and pig metal being then both superior to what they were at any other time. The same furnace manager, Mr. B. Perry, who had the care of the Pottsville furnace during its prize blast of ninety days' continuance, and who likewise blew in the Roaring Creek furnace, which succeeded from the first moment of its action, had charge of the Columbia furnace during the period above referred to; so that its want of immediate success cannot be attributed to inexperience ; and as all the materials are essentially the same in kind as those used at Roaring Creek, we are compelled to believe that want of sufficient blast was the main cause of the little success which attended the first trial at this establishment.
The consumption of stock and yield of pig metal, recorded in the table, were taken from the records of the establishment for the month of May, 1841. It will be seen that the ore required per ton of pig was nearly 3.00 tons;
Anthracite per ton of pig was nearly 2.58 tons
Limestone per ton of pig was nearly 2.03 tons
Air per ton of pig was nearly 20.4 tons.
On the 27th of July 1841, the Columbia, furnace was in active operation and making excellent grey foundry iron, and on that day I took the observations relative to the volume pressure and temperature of the blast recorded in the table.
The large proportion of anthracite used to make a ton of pig, at these works, was accounted for by the proprietors, by stating that at the time to which these notes refer, they were using a considerable quantity of Shamokin coal, which they represented to be much inferior to that of Wilkesbarre, even alleging that two tons of the latter were, for all purposes at the furnace, equal to three of the former. The very large proportion of limestone will also appear singular to those who reflect that the ore itself is calcareous, being in part derived from that portion of the fossiliferous bed which has not been exposed to the decomposing influences of the air. The correctness of the judgment in regard to the anthracite of Shamokin, will no doubt be, ere long, put to a full test in the fine establishment No. 10, of the table, which is now nearly completed.
The force of engines computed from the quantity of air furnished and the pressure under which it is supplied, show that the power required at the Columbia is that of 29.7 horses.*
Thus air, compressed with 3 lbs. per square inch, is subjected to a total pressure of 18 lbs., or 1-1/5 atmospheres, or 6/5. atmosphere; and its bulk will, accordingly be 5/6 as great as, when only under ordinary atmospheric pressure. The bulk of 1861 cubic feet, supplied by the machine in one minute, will become, when compressed, 1550 cubic feet. The compressing power expended, before any air escapes from the blowing cylinder, is very nearly one half as great as would be required to force out the 1/6 of air after compression, which occupied the space through which the piston moved before any began to escape through the valve. Hence the total mechanical force will be 11/12 of what would be required to expel the cylinder full if originally under the pressure of 6-5 of an atmosphere. Thus, 11/12 x 1861 = 1705 cubic feet, are to be put in motion under a pressure of 144 x 3 = 432 pounds per square foot; and 1705 x 432 = 736,560 pounds moved one foot or raised one foot high in a minute. Adding to this 1/3 for friction, and dividing by 33,000, (the pounds raised one foot high per minute, which represents a horsepower we get
(736,560 + 245,520)/ 33,000 = 29.7 horse power.
8
and 9. MONTOUR FURNACES.
These two-furnaces having lately gone into blast, it is not practicable to deduce from their operations any very certain results, as the data relative to the charging, yield and operation of the works were obtained on the 27th of July, 1841, only sixteen days after the first was put into blast. The second, (No. 9,) is understood to have been since put into operation, and both to be performing well. The pig metal, obtained on the day when the establishment was visited, was good grey No. 1, and the cinder indicated an easy working. The coal used was. partly from Wilkes-Barre and partly from Shamokin, the latter having been but very recently employed, and its efficiency therefore not adequately tested.
These furnaces are admirably situated in regard to the ore, two valuable beds of which underlie the base of the stacks; and being on a great line of public works, have little to fear from the suspension of operations owing to want of means, of transporting their coal. The plan adopted of using 4 blowing cylinders has, it appears to me, little to recommend it on the score of either economy or convenience. The blast used at a pressure of 4 lbs. to the square inch is also of questionable economy, involving a great loss at all the crevices and joints of the apparatus. . The furnace which was in action at the period of my visit was certainly performing well, so far as the nature of the product was concerned. It should be mentioned that the proprietors of these works are erecting a large rolling mill at Wilkes-Barre, where they intend to puddle iron with anthracite.
10.
SHAMOKIN FURNACE.
The Shamokin furnace can as yet furnish no data for judging of the efficacy of the arrangements there adopted, but by observing the ample provision for power, that of two high pressure steam engines of 80 horse power each, having 10 boilers of 30 feet long and 30 inches in diameter, with steam cylinders 20 inches in diameter and 6 feet length of stroke, working under a pressure of 70 pounds to the square inch, it is evident that all which blowing machinery can do to insure success may be confidently expected in this establishment. The engines are the same which were put up and used at the coke furnace in Farrandsville, already referred to.
The air-heating apparatus consists of three heating ovens, with 15 Calder tubes to each, and four others with 20 tubes each, making 125 tubes in all. This apparatus for blowing and heating air is intended for two furnaces, of the dimensions recorded in the table. The coal will be brought to the furnace from mines but a few hundred yards distant, and the ore may be found within half a mile of the same point. Limestone was also observed within 30 or 40 rods of the stacks. It was originally intended to carry a railroad, to the trunnel head, but the elevation of the materials by machinery has more recently been decided on and the road accordingly laid to the base of the furnace. A second furnace is in progress.
The works now under review will probably be found to solve completely the question of the profitable operation of anthracite establishments on a large scale, and in situations analogous to those of the great iron works of Britain. With regard to their steam machinery it may probably be found that something is yet to be done to economize heat. The enormous waste by radiation, especially from' steam pipes and cylinders, should be guarded against; and if the gas from the steam furnaces be found to escape into the chimney at too high a temperature, a more economical form of 'boiler should be adopted. The subsequent, developments of this essay will be found to justify this opinion. A low pressure engine, to be worked by the escape steam of the two high pressure ones now built, may possibly be found no bad addition in connexion with a foundry or a new furnace.
As both grey and red ash coals are found at Shamokin, the relative values of the two in the blast furnace may be tested, as also the opinion, already referred to, of the value of Shamokin coal in general, as compared with the anthracite of other districts. This latter point may be the more readily settled in as much as the ore-used at first will be that of Danville, in connexion with which this coal has heretofore been employed. Exchanges of coal for ore, and the contrary, ton for ton, have already been made between the Danville and Shamokin companies.
The construction of the Shamokin furnace will be understood by the annexed sketch exhibiting, on a scale of 10 feet to 3/4 of an inch, the interior form and foundation of the stack.
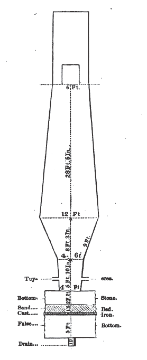
11,12,13,14. STANHOPE FURNACES.
The works at Stanhope, Morris county, New Jersey, are, in some respects the most interesting of all the anthracite iron furnaces in this country. They stand near the summit level of the Morris Canal, between Easton on the Delaware, and Newark on Raritan Bay. By this canal, the coal from Mauch Chunk, Beaver Meadow, Hazleton, Sugar Loaf, Buck Mountain and Summit Company's mines may arrive on the bank, at the level of the trunnel head of these furnaces. The waters of Rockaway river, with a fall of more than 50 feet, are used for the moving power, and the magnetic ore, found in such abundance in that part of New Jersey, is exclusively employed at this establishment. This last circumstance is what gives the highest interest to the Stanhope works. To reduce, in a blast-furnace and without admixture of other ores, this rich mineral, by the aid of anthracite, is what others had hardly dared to hope. The complete success of the undertaking constitutes an era in the business, of which the Stanhope company may justly be proud; an era, perhaps, equally important to the two states which respectively furnish the ore and the anthracite.
The
greatest amount of iron which had been made per day, previous to the 12th of
June, 1841, when these works were visited, was 9.23 tons, or 64.63 tons per
week, which was not however continued for many days in succession. On the 11th
of June, the product was 14,644 Ibs., and on the 12th, 14,630; or at the rate
of 45.7 gross tons per week. A slight derangement of the conducting tubes had,
it was said, caused a temporary falling off in the yield for two or three days.
Fifty-six tons per week was stated to be the average product. The iron was not
remarkable for toughness, though very soft, and probably a re-melting in the
cupola would improve its quality as cast iron.
The
variety of anthracite preferred to others at these works is that of Beaver Meadow.
Coal has been delivered at the works at four or four and a half dollars per
ton.
15. SCHUYLKILL VALLEY FURNACE.
This
furnace has been put into blast since a part of the foregoing pages were sent
to press, and it is not therefore practicable to do more than refer to casual
statements which have reached us relative to the immediate success of its
operations. Its situation is certainly not less favorable in regard to
materials than that of the Pottsville or Shamokin furnaces. In reference to
ore, it is probably as advantageously located as any others, except perhaps the
Shamokin and Danville establishments.
GENERAL COMPARISONS.
In
order to compare the supply of air and the yield of iron, with the area of
cross section of each furnace at the boshes, and the power employed, with the
yield in pig metal per week, the following table has been constructed from the
data furnished by the table at pages 28—31.
TABLE 1I.
|
Name of furnace. |
Diameter of boshes |
Area of boshes (sq. ft.) |
Yield og pig material per week.
(Tons) |
Area of boshes perr ton per week |
Air blown per sq. ft. of bosh per
minute. |
Air furnished by cylinders (CFM) |
Power req'd to inject air (hp.) |
|
Mauch Chunk |
5.5 |
23.758 |
8 |
2.970 |
29.47 |
700 |
7.66 |
|
Pottsville |
8.75 |
60.132 |
28 |
2.148 |
62.68 |
3769 |
31.4 |
|
Roaring Creek |
8.5 |
56.745 |
40 |
1.419 |
42.29 |
2400 |
32.4 |
|
Roaring Creek |
8.5 |
56.745 |
35 |
1.621 |
28.94 |
1672 |
12.8 |
|
Phoenixville |
8 |
50.265 |
28 |
1.795 |
34.45 |
1732 |
14.16 |
|
Danville |
7.5 |
44.179 |
35 |
1.262 |
54.63 |
2114 |
35.6 |
|
Columbia |
8.5 |
56.745 |
31.5 |
1.801 |
32.79 |
1861 |
29.7 |
|
Montour |
12 |
113.097 |
70 |
1.616 |
44.44 |
5026 |
104.6 |
|
Craneworks |
12 |
113.097 |
50 |
2.262 |
44.78 |
5065 |
68.3 |
|
Stanhope |
10 |
78.540 |
45.7 |
1.719 |
51.83 |
4071 |
65.1 |
*On examining and
comparing this result with others, there seems to be reason to suspect that
some error has been made in either observing or noting the number of strokes of
the pistons in the blowing and steam cylinders at the Columbia furnace. As
recorded in Table 1, The speed of
the former is but 5-8ths that of the latter. It seems probable that this proportion
should have been reversed. If so, the blowing cylinders will make 25. double
strokes, each per minute instead of 15 3-8ths. The bulk of air will then be
2977 cubic feet per minute instead of 1861, and the air per square foot of
bosh, will be 52.49 cubic feet—more nearly corresponding with that of the
Danville furnace,—and the power will be that of 47.5 horses.
The
formula employed for computing the power required to inject the bulk of air
given by observation, and under the pressure noted, is
.00576 p {15 A /(15+p) + (A - [15 A/(15 + p)])/2}
=
the horsepowers of the blowing machine, whether water or steam. Here A = the
bulk of air in cubic feet per minute, and p = the pressure in pounds per square
inch, within the blowing cylinders. The horsepower is that of Watt, and the
allowance for friction, &c. is supposed to be one fourth of the whole, or
one third as much as is required to compress and inject the air.
The
initial pressure of the air is assumed to be 15 pounds to the square inch, and
the temperature 60¡. It would be as yet a misplaced refinement to enter into
minute computations relative to the quantity of moisture in the air, and the
variations of force required in the machinery on this and similar accounts, at
different seasons of the year. When with a limited power only at command, it is
desired to inject an increased volume of air, the obvious expedient is to
enlarge the area of the nozzles, and thereby reduce the pressure and increase the speed. When, at each stroke, a space
is left above and below the pistons in the blowing cylinders, from which the
air is not expelled, the recoil of this residual air after compression
obviously causes it to occupy some portion of the space which would otherwise
be filled by air newly admitted. Hence the importance of accurate adjustment
between the piston and cylinder heads. The piston rods of blowing cylinders
sometimes pass "through and through" both heads, especially when the
cylinders are laid horizontally; and then the bulk of air taken in at each
single stroke, is the same; but as this construction is not adopted at any of
the anthracite works, a deduction is to be made of a few feet per minute for
the bulk of the piston-rod filling a portion of the space on one side only at
each revolution.
From
the preceding table, it appears that great differences exist between the
quantities of air required to be blown through a given section of boshes per
minute; but that the average supply, including the two modes of driving Roaring
Creek furnace, is 42.63* cubic feet
of air to one square foot of bosh, but as this includes all the air blown, as
well for heating ovens as for the furnace itself, the quantity taken by the
latter will be considerably less. The iron made per minute by each square foot
of boshes, is, on an average, 0.127 pounds, or 182 7-8 pounds in twenty four
hours. Hence, the bulk of air required to pass the bellows in making 1 pound of
iron, is 335 2-3 cubic feet, or 25 4-5 pounds.
With
regard to the area of cross section at the boshes, as affecting the amount of
iron made, it appears, that if we omit the Mauch Chunk furnace, and take
Roaring Creek as making 40 tons per week, the production of one ton of pig iron
per week, is derived from 1 3-4 square feet of bosh,—or one ton per day from
every 12-1/4 square feet.
* By the
supposition in the preceding note, (page 66) this number will be raised to
45.13.
Comparing
the whole number of tons produced per week, by all the furnaces with the whole
amount of power by which the blast appears to have been furnished, we find
1.08* horsepowers, as the force employed in giving one ton per week. If this
result be increased to 1-1/4 horse power, it may, I think, be relied on as an
entirely safe basis for calculations in the construction of blowing machinery for
anthracite works, and give a surplus sufficient to answer in all emergencies.
*Or 1.13, in
accordance with the note on page 66.
The
average force or pressure of blast, appears to have been 2.4 pounds per square inch,
and though it has often been attempted to employ a blast greatly inferior to
this in tension, I am not aware of much success having attended these attempts.
Either a falling off in the yield, an inferiority of metal, or a destruction of
the furnace hearth has usually been the consequence. It is evident, that the
amount of power required to inject the air will diminish in direct proportion
to the decrease of pressure; but until some evidence more conclusive than what
has hitherto transpired, shall be adduced in favor of a softer blast, it seems
better to adhere to what is now giving good results in several of the
anthracite furnaces.
CHARACTER AND CONSTITUTION
OF ANTHRACITE—AMOUNT OF BLAST REQUIRED FOR ITS COMBUSTION.
In the
prosecution of the manufacture of iron, with any kind of fuel whatever, it is
desirable to know in advance, at least within approximate limits, what amount
of mechanical power will suffice to administer in the most advantageous manner,
the requisite quantity of air to the furnace.
Two
circumstances will chiefly determine this question.
First, the weight of oxygen required from the air, for the
complete combustion of the fuel to be used in a given time.
Secondly, the pressure under which it is to be delivered to
the furnace.
In
the case of anthracite, the weight of oxygen will, in general, be easily
computed, since it contains little or no other combustible than carbon, and
since the quantity of this is pretty well ascertained, for the various coal fields
which supply iron furnaces.
Analysis
of single, well selected specimens of anthracite, must not, however, be too
implicitly relied on. There is, inevitably, intermixed with the coal, more or
less slaty matter, or coal of a semi-combustible character, which allows it to
pass almost unchanged through the blast furnace. This, as well as the earthy
residuum of the coal itself, is to be deducted, together with the volatile
matter, before assigning the quantity of carbon which is to undergo combustion
in the blast furnace. It will not be far from the truth to deduct for volatile
matter, ashes and unconsumed coal or slate, 15 per cent. of all the anthracite
which is put in at the trunnel head, leaving 85 per cent. for the carbon
consumed. Some varieties will doubtless give a small per cent. more than this
quantity, while others will yield less. To make the computation more nearly
accurate, a large quantity of the particular anthracite used, should be
analyzed, and the quantity of earthy matter after incineration, be carefully
weighed. If, in use in a furnace, the amount of unburnt slate and coal which
comes through in a given time, should be ascertained.
The
results obtained by calculations of the kind here indicated will of course give
only approximations. The ore will furnish no inconsiderable quantity of oxygen.
Some atmospheric air will escape combustion; and much of the gas escaping at
the trunnel head is not carbonic acid, but carbonic oxide and carburetted
hydrogen.
To
aid in forming estimates of the volume of air required in anthracite furnaces,
the following analyses of that material, from different parts of the coal
regions, may be consulted.
Though
not immediately connected with our present investigations, yet for the purpose
of ready comparison, it has been deemed proper to add, in a subsequent table,
some analyses of our free burning bituminous coals.
TABLE III.
View of the composition of
some of the anthracite coals of Pennsylvania, a' determined by the writer's
analyses.
|
|
Locality of Coal |
Sp. Gr. |
Vol. Matter. |
Carbon. |
Ashes. |
|
1. |
Summit Co.'s Lands, head of
Beaver Creek |
1.560 |
6.42 |
97.30 |
1.28 |
|
2. |
do. 2d bed |
1.594 |
4.31 |
91.69 |
4.00 |
|
3. |
do. 3d bed |
1.613 |
7.51 |
87.48 |
5.01 |
|
4. |
do. 4th bed |
1.630 |
9.60 |
85.34 |
5.06 |
|
5. |
Stevenson's Bluff, west of
Beaver Meadow |
1.613 |
9.23 |
86.06 |
3.71 |
|
6. |
Buck Mountain |
1.559 |
5.90 |
91.02 |
3.08 |
|
7. |
Sugar Loaf Co., 1st specimen |
1.591 |
6.98 |
88.19 |
4.83 |
|
8. |
do. 2d bed |
1.574 |
5.36 |
85.91 |
8.73 |
|
9. |
do. same bed, but further
down the slope |
1.550 |
6.87 |
90.71 |
2.42 |
|
10. |
Lyken's Valley, 1st sample |
1.391 |
7.60 |
87.95 |
4.45 |
|
11. |
do. 2d sample |
1.404 |
5.95 |
89.30 |
4.75 |
|
12. |
do. 3d sample |
1.416 |
10.00 |
85.70 |
4.30 |
|
13. |
do. 4th sample |
1.374 |
4.60 |
88.70 |
6.70 |
|
14. |
do. 5th sample |
1.376 |
8.35 |
87.75 |
3.90 |
|
15. |
do. 6th sample |
1.395 |
8.30 |
88.65 |
3.05 |
|
16. |
do. 7th sample |
1.382 |
8.65 |
87.20 |
4.15 |
|
17. |
do. 8th sample |
1.398 |
11.85 |
84.00 |
4.15 |
|
18. |
do. 9th sample |
1.378 |
7.30 |
87.00 |
5.70 |
|
19. |
Mauch Chunk, Summit Mines |
1.590 |
7.90 |
87.10 |
5.00 |
|
20. |
Room Run Mines |
1.604 |
6.15 |
87.20 |
6.65 |
|
21. |
Pottsville |
1.569 |
6.71 |
86.54 |
6.75 |
The
first nine of the above analyses, give a fair average of the coal at the
eastern extremity of the middle coal field, and show that the volatile matter
is 6.91, the fixed carbon 88.744, and the ashes, 4.346 per cent. The mean
specific gravity of these nine varieties is 1.587. The second nine give the
character of the north-western termination of the southern anthracite field.
The mean percentage of volatile matter is here 8.066, of carbon 87.36, and of
ashes 4.574.
The
amount of these several ingredients in the last class of coals, is nearly
identical with those of the anthracite used by Mr. Crane, in his iron works in
South Wales.
TABLE IV.
View of some of the "free-burning"
bituminous coals of Pennsylvania, suitable to be used in blast furnaces, either
with or without coking.
|
|
Locality |
Sp. Gr. |
Vol. Matter. |
Carbon. |
Ashes. |
|
1. |
Savage Mountain Coal trough
Somerset County |
1.319 |
20.20 |
75.75 |
4.95 |
|
2. |
do. 2d bed |
1.321 |
29.90 |
69.10 |
11.00 |
|
3. |
do. 3d bed |
1.343 |
21.80 |
69.90 |
8.10 |
|
4. |
do. 4th bed |
1.362 |
19.80 |
68.54 |
11.66 |
|
5. |
do. 5th bed |
1.363 |
18.30 |
71.50 |
10.20 |
|
6. |
do. 6th bed. |
1.370 |
18.80 |
70.70 |
10.50 |
|
7. |
do. 7th bed |
1.386 |
20.10 |
68.46 |
11.44 |
|
8. |
do. 8th bed |
1.388 |
19.50 |
68.44 |
12.06 |
|
9. |
do. 9th bed |
1.480 |
18.70 |
68.56 |
12.79 |
|
10. |
do. 10th bed |
1.491 |
17.60 |
66.36 |
16.04 |
|
11. |
do. Maryland Mining Co.
(Maryland) |
1.437 |
15.62 |
68.56 |
15.82 |
|
12. |
do. George Creek at
Lonakoning, (Maryland.) |
1.346 |
16.03 |
70.75 |
13.22 |
|
13. |
Carbon Creek, Bradford Co.
(Pa.) 1st sample |
1.515 |
15.00 |
62.60 |
22.40 |
|
14. |
do. 2d sample |
1.448 |
17.40 |
70.00 |
12.60 |
|
15. |
do. 3d sample |
1.465 |
19.10 |
63.90 |
17.00 |
|
16. |
do. 2d bed, 1st sample |
1.377 |
20.50 |
68.10 |
11.40 |
|
17. |
do. 2d bed, 2d sample |
1.378 |
19.20 |
65.50 |
15.30 |
|
18. |
do. 2d bed, 3d sample |
1.349 |
19.30 |
74.97 |
5.73 |
|
19. |
do. 3d bed, 1st sample |
1.388 |
17.90 |
69.00 |
13.10 |
|
20. |
do. 3d bed, 2d sample |
1.400 |
18.90 |
68.57 |
12.53 |
|
21. |
Lick Run, Lycoming County,
(Pa.) Diamond ply |
1.320 |
19.80 |
75.20 |
5.00 |
|
22. |
Quinn's Run, Lyco,ing County |
1.373 |
18.80 |
74.40 |
6.80 |
|
23. |
Braod Top Mountain, Bedford
Co. (Pennsylvania) |
1.301 |
15.90 |
77.60 |
6.50 |
It
appears from Table I. that the number of tons of anthracite supplied per week
to seven furnaces, viz., Roaring Creek, Phoenixville, Danville, Crane, Columbia,
Montour and Stanhope, is 501.3; and as these furnaces make 310.5 tons of pig
metal per week, and demand, on an average, 4.5 cwt. of anthracite to each ton
of pig for heating their blast, their total weekly consumption will be 571.16
tons. Hence the anthracite demanded for both smelting and heating blast is
571.16 / 310.5 = 1.84 tons = 1 ton 16 cwt. 5 qrs. 5.6 lbs. to the ton of pig
metal produced. If this anthracite were pure carbon and were completely
converted into carbonic acid, the weight of oxygen required for that purpose
would be 16 X 1.84 = 4.906 tons; but if we admit that the mean of the two sets
of analyses above given represents the average quantity of carbon in
Pennsylvania anthracite, viz., 88 per cent., then the quantity of oxygen will
be but 88 / 100 * 4.906 4.317 tons. As the oxygen is to be supplied from the
atmosphere, of which the composition, (omitting moisture and impurities,) is 28
parts by weight of nitrogen to 8 of oxygen, the total quantity of air for one
ton of pig will be 36 / 8 X 4.317 = 19,426 tons; which, at 13.22 cubic feet to
the pound avoirdupois, will be equal to 572,255 cubic feet. Hence it is easy to
calculate what number of cubic feet of air should be delivered to the furnace
and heating ovens, when we have determined how many tons of iron can be made
per day. Thus, suppose the furnace to make 7 tons per day, the time for making
one ton will be 1440 / 7= 205.7 minutes, and the number of cubic feet of air required
to pass the nozzles in one minute
will be 579,255 / 205.7 = 2782.
The
seven furnaces above named receive 22,569 cubic feet of air into their blowing
cylinders per minute, and the aggregate area of their boshes is 512.67 square
feet. The anthracite which they use will not probably yield over 85 per cent.
of pure carbon, after deducting that which escapes combustion and comes out
with the cinder, together with the slate unavoidably intermixed, and the dust
which is projected out at the trunnel head. The quantity of anthracite which
makes one ton of pig is, as above, 1.84 tons.
The
time required for the seven
furnaces to make one ton of pig is 17,640 / 512.67 = 34.4 minutes. The weight
of carbon burnt in that time is .85 x 1.81—1.564 tons; and this will
require 2.66 times its weight of oxygen to form carbonic acid, or 4.16 tons.
This quantity of oxygen will be contained in 36 X 4.16 = 18.72 tons of air;
which, at 29,612.8 cubic feet to the ton, gives 551,351.6 cubic feet of air to
make one ton of pig; and as this takes 34.4 minutes, the air required to be burnt
at all the furnaces per minute, is 554,351.6 / 34.4= 16,149 cubic feet.
Deducting this from 22,569, the quantity derived from observations on the
movements of the blowing pistons, we have a surplus of 6,454 cubic feet, or
28.4 per cent. of the whole, either not completely expelled through the
eduction valves, or allowed to escape at the safety valves, joints and tuyeres,
or remaining unburnt in the furnace and heating ovens. With regard to the
latter, it may be safely asserted that they do not consume more than one half
of the oxygen which passes through their grates.
In
general, according to a preceding deduction, let the number of tons of iron
which a furnace can make per day be represented by B/12.25; B being the area of cross section of such
furnace at the boshes. Then will the time, in minutes, of making one ton be
12.25 / B x 1440 = 17640 / B. Let
the proportion of carbon in 100 parts of the anthracite used be c /100, and the
weight of anthracite, in tons, required to smelt one ton of pig be a; then the
quantity of carbon consumed in making a ton of iron will be ca/100, and the
weight of air, in tons, required for its combustion into carbonic acid, 16 / 6
x 36 / 8 x ca / 100 = 0.12ca The conversion of this expression of the weight of
air into cubic feet, is easily effected, since 13.22 cubic feet weigh one pound
avoirdupois, and the ton of air consequently contains 2240 x 13.22 = 29,612.8
cubic feet. Hence the number of cubic feet of air used in making a ton of pig
metal will be represented by 29,612.8 x 0.12ca = 3553.5ca. Dividing this bulk
of air by the above expression, representing the time in minutes required to
make one ton of pig, we get 3553.5ca / 17640 / B = .2014caB = the number of
cubic feet of air required per minute by the furnace and heating ovens. In
other words, multiply together the area of boshes in square feet,—the
weight of anthracite in tons used per ton of pig, produced,—the number
representing the per centage of carbon in the anthracite and the decimal 0.201
1, and the product will give the number of cubic feet of air, before
compression, which must enter the furnace and heating ovens per minute. If we
take into account the quantity of oxygen contained in the ore, it might be
supposed that a large deduction would be allowable from the bulk of air given
by this formula; but the quantity of oxygen which does not undergo combustion
will account for the fact, that even a greater quantity than that given by
calculation is actually injected into the fires.
It
will of course be understood, that all the above deductions are to be regarded
as approximations only, such as the present working of the several
establishments enables us to make. To give exact data for calculations of this
nature, they ought to be furnished with more correct instruments for observing
and recording the several items which enter into the computation. The waste
space above and below the piston should be known. The number of movements of
piston per day should be marked by a self-registering apparatus; the pressure
should be marked by an inverted syphon gauge of large-sized glass tube; the two
limbs being accurately of the same diameter, and connected at bottom by a
section of diminished and almost capillary size, thus preventing rapid and
violent oscillations, which always interfere with accurate experiments. Where
works are situated at considerable elevations, the mean barometric pressure
should be known,—and if the season of making observations do not extend
through the year, the temperature and dew point of the air at the times of
observing, should be reduced to that of the annual mean. It would be desirable
to know, in all cases, the quantity of matter volatile at a white heat, both in
the ore, the coal, and the limestone; as well as the fixed matter, other than
iron, in the ore, the ashes of the coal, and the lime or other materials after
calcination in the limestone. The weight of cinder as well as of pig metal,
which is drawn from the furnace, should be ascertained, if we would form a just
and intelligent estimate of what is going on within. Due economy of moving
power, is every where more or less important, and hence the accuracy of
workmanship in blowing apparatus, can hardly be over-estimated. Where
anthracite is transported to a distance for supplying this force, the best
means of applying its heating power should be well understood. Great economy
has within a few years been obtained by an attention to philosophical
principles, in generating and using steam, whether obtained from wood or from
mineral fuel; and since the most wasteful practices often exist in connexion
with this part of an iron establishment, a careful attention should be given to
ascertain the quantity of water by weight which goes into the boiler, per week,
as well as its temperature and the weight and quality of the anthracite with
which the evaporation is effected. The evaporative power of anthracite, that
is, the number of pounds of water which can be vaporized by the combustion of
one pound of the fuel, has already engaged attention, and is likely to be still
more minutely examined. Among the causes which interfere with the economical
action of steam boilers, is the want of sufficient heating surface in the
boiler, compared with the quantity of steam which it is required to supply, and
the consequent necessity of urging the draught to such a degree as to carry
away a great portion of heat in the gases which escape into the chimney. The
use of high pressure steam, without condensation, of course involves the loss
of at least one atmosphere in the total pressure generated. So important is the
subject of the heating and evaporating power of anthracite to the iron master,
as well as to the manufacturer and to the navigator by steam, that no apology,
will be required by the reader for our introducing the following remarks in
relation to this subject.
EVAPORATIVE POWER OF ANTHRACITE.
Writers
have heretofore stated, that when bituminous coal is submitted in gas retorts
or coking ovens, to such a temperature as to deprive it of a large portion, or
the whole of its volatile matter, it still retains nearly the same heating
power in the form of coke, which it had possessed in that of coal.
Thus,
in his paper on the evaporative power of coal, in the transactions of the
Institution for Civil Engineers, Vol. 2, p. 159, Mr. Josiah Parkes, says,
"I have myself invariably found, as might be expected, that species of
coal to be the strongest fuel, which contained the least gas, and vice versa."
"
I have also found that 75 pounds of coke produced from 100 pounds of coal,
evaporated as much water as 100 pounds of the self-same coal."
When
burning coal yielding 34 per cent. of volatile matter, Smeaton found that its
coke would produce, on the same grate, 83-1/3 per cent. as much effect as an
equal weight of the coal; but it is probable that had the grate been adapted to
coke, the effect of the latter might have been still more favorable.
Mr.
Apsley Pellatt's experience, in a glass furnace is cited by Mr. Parkes, as
follows:
"Mr.
Pellatt's mode of burning coke exhibits, in a far more perfect manner than any
steam boiler can do, the relative calorific value of coke and coal. The space
within his glass pot furnace, gives abundant room for the combination of air
with the gaseous products; the flames are not extinguished by comparatively
cold surfaces like those of a boiler, which, after inflammation, reduce them
back again into smoke; the heat requisite for perfect combustion is always
present, and his furnaces are particularly favorable to the development of all
the power of coal; yet he finds common gas coke to be superior to coals in heating power by 25 per
cent.; and gas coke is stated by M. de Pamhour to be found inferior to Worsley
coke by 12 1-2 per cent., which no one acquainted with coke will doubt; thus
exhibiting an excess over coal of 37 1-2 per cent."
Mr.
Wood, in his treatise on railroads, states his experiments on locomotive
boilers with coal, to have given a result of 4.46 pounds of water evaporated
from 60¡ by the consumation of 1 pound of fuel; while M. de Pambour from the
mean of eleven experiments on locomotive engines, burning coke, shows that the
evaporative power of the latter is 6.21 pounds of water to 1 of coke, thus
indicating a superiority of nearly 40 per cent. in favor of coke over coal.
From
a temperature of 212¡ Mr. Wood's coal would have evaporated 5.12 lbs. of water,
and M. Pambour's coke, 7.12 lbs. It should, however, be mentioned that in this
case, the whole deficiency not probably attributable to the inferiority of coal
to coke, but in part also to the want of sufficient absorbing surface. In Mr.
Wood's experiments, this was only 9.61 square feet to one square foot of grate;
while in M. de Pambour's it was 47.6 feet, or nearly 5 times as much.
A
knowledge of the superiority of the fixed over the volatile constituents of
coal, induced the writer, in 1838, to compute and publish in the National
Gazette, of Philadelphia, the relative value of some of the anthracites of
Pennsylvania, and the bituminous coals in use in this and other countries. This
superiority in economy for naval purposes, was predicated on two circumstances.
First, the superior efficiency of
anthracite, weight for weight; and, second, its greater specific gravity, by which a greater weight may be stowed
in a given amount of space on shipboard. For the purposes of the iron masters
and manufacturers in general, who use this fuel, for producing steam, the first
consideration alone is of much importance; but this, together with questions in
regard to the most economical method of burning it and applying its heat, will
be found of great interest in a course of years, even where anthracite is to be
had at the lowest rate.
If
it can be shown that, by a judicious arrangement of boilers and grates, the cost
of one or two tons of coal per day can be saved to an iron furnace,—this
amount, trifling as it may seem, where coal does not cost more than one dollar
per ton, at the works, may still be found to constitute the interest of a
pretty large sum at the end of a year. One dollar per day is more than enough
to pay the interest on the entire cost of the engine and boilers, at some of
our large iron works, and certainly would amply compensate for any increased
expense of boiler, which might be found necessary, in order to apply correct
instead of erroneous principles of combustion.
The
actual evaporative power of any fuel as determined by practice must depend both
on the nature and constitution of the fuel, and on the kind of arrangement
adopted to effect its combustion, whether slow or rapid, and to apply its
calorific energies. Hence the importance of knowing the form of boiler, size
and construction of grate, and the extent and position of heat-absorbing
surfaces best adapted to give high evaporative results.
In
all the iron works, using steam power, to which reference has been made in the
preceding pages, the kind of boiler used is the ordinary simple cylindrical
one, having neither side nor interior return flues, and consequently allowing
no greater average circuit to the heated gases than from the centre of the
grate to the entrance of the flue, of course less than the length of the
boiler. It may on an average be computed that the absorbing surface of each
boiler is one half its curved surface. Some of the experiments, which will be
hereafter cited, will show how little economical such a boiler is, as compared
with other forms and arrangements which might be adopted.
Among
the earliest of those who have studied this subject with a view to its useful
applications, may be mentioned the celebrated -- Watt, who at the Albion Mills,
and in a wagon boiler, of the form usually adopted by him, obtained the result
of 3.62 pounds of water evaporated from its initial temperature, or 9.63 from a
temperature of 212 deg. by the combustion of one pound of Newcastle bituminous
coal. In later times the observations of Mr. Lean on the performance of the
Cornish engines used in pumping, have put us in possession of numerous and
valuable facts in regard to the evaporative power of the same species of fuel
found in Wales. At first the effect of the bushel of coal was measured by the
performance of the engine, to which the steam was administered, thus
complicating the question of the production of steam with that of its application. More recently, however, a method has been devised
for determining and registering through the agency of an apparatus which may be
termed an aquameter, the quantity
of water delivered in any given period to the boiler. By means of this
registration it has been ascertained, that in the Cornish double cylindrical
boilers, 36 feet long, with an exterior shell 6 feet; and an interior one of 4
feet in diameter, and affording to the flame or hot gas, a circuit of 172 feet,
or a little more than four times the length of the boiler, the effect of one
pound of bituminous coal is the evaporation from a temperature of 212¡ of 11.62
pounds of water.
An
opinion prevails to some extent in this country, that the locomotive boiler is
among the most economical forms of evaporating vessels; but Mr. Parkes has
proved that where a pound of coal is burned in 44.03 seconds in a Cornish
boiler, it produces more than twice the evaporative effect of the same weight
of fuel burned in 6 3-4 seconds in a locomotive boiler; and a series of
experiments made under the directions of Mr. Stevens, at Bordentown, N. J.,
also in a locomotive boiler, used at the time for stationary purposes, has
proved that whether wood or anthracite be the fuel, an increase in the rapidity of combustion in the same boiler, is
accompanied by a diminution in the
evaporative efficiency of the combustible. It has also shown that the rate of
diminution in evaporative effect, is within certain limits more rapid than that
of the increase of combustion, in the ratio of 4 to 3. Messrs. Parkes and
Manby's experiments on board the steamer " Anthracite," with Player's
boiler, using anthracite coal for fuel, amply demonstrate the same general
truth.
Many
of the experiments of Dr. Dana hereafter cited, will be seen clearly to prove
the truth of the position, that beyond certain limits an increase in the rate
of combustion in any given boiler is attended with a loss of useful effect.
It
is evident that the heat-absorbing surface of a steam-boiler might be so great,
and the circuit to be traversed by the heat so extended, in comparison with the
quantity of combustion taking place on its grate, that the hot gaseous matter
would not escape until some time after it had imparted all the heat it was
capable of yielding to the fluid within the boiler. In such case the gas
remaining at a uniform temperature for the latter periods of its transit, would
clearly not have its efficiency diminished by such an increase of combustion as
should urge it with more rapidity towards it exit from the boiler. On the
contrary, a farther diminution in the rate of combustion might allow the
radiation and conduction of heat a greater length of time to exercise their
influence in diminishing the
evaporation. When the rapidity of combustion is such as to send the products of
combustion beyond the absorbing surfaces, at a temperature greatly above that
of the steam in the boiler, it is evident that some loss must be the
consequence, for the escaping gas would then, if applied to a boiler within the
chimney, evidently be able to generate an additional portion of steam of the
same tension, since we know of no limit to the principle that a hotter body
will impart heat to a colder.
Whenever,
in the progress of combustion, apertures of considerable magnitude occur among
the fuel, large portions of unburnt air make their way through the fire, and
not only prevent the latter from doing its office for the time, but become
robbers of the more useful portions of air by depriving the burning mass of its
heat, which are taken away into the chimney, and dispersed at the top. All who
have witnessed the effect of burning anthracite-dust with a fan blast, will
have noticed the constant tendency to form little blow holes, which, enlarging
by degrees, allow portions of air to become heated excessively, but not burnt.
A portion of the dust is likewise projected upwards, and sent wholly beyond the
seat of combustion. When coal of a large size is burned in too thin a stratum
on a grate, many interstices must in like manner exist, and, in the case of
dust and pea coal, the superiority of effect arising from mixing with them a
portion of bituminous coal, will probably be found to depend on the partial
agglutination and envelopment of the particles of anthracite - in those of the
coke, preventing the mobility of the former, and compelling the air to a more
minute subdivision and equal distribution throughout the mass. In regard to the
quality of the dust of anthracite, it may in general be regarded as quite equal
in purity to that which comes to market in larger masses, since the brittleness
of pure anthracite, and the toughness of slate, allowsthe former to be more
comminuted than the latter.
On
the subject of the evaporative power of bituminous coal, when employed in
boilers of different forms, we have several elaborate papers by Mr. Josiah
Parkes, published in the Transactions of the Institution of Civil Engineers.
The table contained in the 3d vol. of that work, page 43, is particularly
interesting on account of the numerous facts which it embodies, and of the
conclusions to which a comparison of these may lead. A few of the data there
furnished, will enable us to institute comparisons between the results of
American experience with anthracite, and that of the English engineers, when
using bituminous coal or coke.
"The
practice of slow combustion," says Mr. Parke, "is evidently conducive
to economy in the treatment of fuel."
"Boilers
tested as to their merit by their respective evaporative economy, arrange
themselves for consideration in the inverse order of the rate of
combustion." * * *
"A
second, though somewhat less regularcoincidence between the operating causes
and economical results, is indicated by the extent of surface exposed to absorb
the heat supplied to the boiler. *
* Economy of heat is
promoted in some proportion of the extent of the absorbing surface."
Mr.
Parkes lays considerable stress upon the thickness of metal of which a boiler
is composed, as influencing the rate of evaporation, and also on the temperature
of combustion, as affecting the durability of boilers. In regard to the former
of these points, I may mention that my own experiments on iron of different
thicknesses, from 1-50 to 1-4 of an inch, and those of Mr. Hayes, from 1-8 up
to 1 inch, prove that within these limits, which are in both directions far
beyond the requisitions of the steam-boiler, no sensible difference will be
produced in the evaporative effect of fuel. And with respect to the second
point, I would refer to the fact, first established by my experiments,
published some years since in the American Journal of Science, and since
reproduced both in England and in this country, that so long as water is in
contact with iron under atmospheric pressure the metal will not receive a temperature
much more than 100¡ Fahrenheit above the boiling point; for, from 312¡ to 324¡
is reached the temperature of maximum vaporization,—a rate of generating
steam far beyond the practice even in locomotive engines.
Another
fact may be mentioned in proof that unnecessary stress is laid on these
particulars, which is, that metallic tubes, of moderate thickness, even of
copper, lead, or soft solder, when kept filled with water, may be used to
traverse a fire where an intense temperature prevails, as in a grate using
anthracite, without danger of melting. Years of experience have convinced me of
this truth. It is also well known that in house-heating apparatus, on the hot
water system, wrought iron tubes of a quarter of an inch or more in thickness,
are made to pass through or around the fire, and yet remain for a long time
without sensible deterioration. I have employed such an apparatus with
anthracite, without the least inconvenience. Bad iron may suffer deterioration
when used for a boiler at any temperature, and corrosive liquids, or gases, may
destroy the best, but it is often observed that in wrought iron heating
apparatus the parts near the fire suffer less than those more remote. When a
boiler is allowed to become coated with sediment, it matters little whether
wood, bituminous coal, or anthracite, be the fuel. It must inevitably suffer
from overheating.
The
Cornish boiler combines the advantages of slow combustion, large relative
absorbing surface, and great length of time for heat to remain in contact with
any given portion of that surface.
MR. PARKES'S RESULTS
In the Cornish Boiler.
"1 lb. of coal was burnt in 44.08 seconds.
3.46 lbs. of coal was burnt on each square foot of
grate per hour.
1 lb. of water was evaporated by 1 square foot of
surface per hour, from 212¡.
11.62* lbs.
of water were evaporated by 1 lb. of coal from 212¡."
Wagon Boiler, Warwick
experiments.
" 1 lb. of coal burnt under one boiler in 38.31
seconds.
4 lbs. of coal burnt on each square foot of grate per
hour.
6.39 lbs. of water evaporated by 1 square foot of
heated surface per hour from 212¡.
10.23 Ibs. of water evaporated from 1 lb. of coal,
burnt from 212¡."
Mr. Parkes, by a trial of 6 months' continuance,
evaporated 18 1-2 cubic feet of water
* By assuming the
latent heat of vapor to be according to the determination of Watt, only 950¡,
Mr. Parkes produces in this case 11.82, and in other cases, corresponding
differences in evaporative results, all which I have adjusted to a latent heat of
1030¡ by 112 Ibs. of coal, or 10.23 lbs. to 1 lb. of coal, from 212¡, as stated
in the preceding extract.
Wagon Boiler, mean of eight
experiments.
"1 lb. of coal burnt under one boiler in 16.57
seconds.
10.75
lbs. of coal burnt per square foot of grate per hour.
7 1-10 lbs. of water evaporated by 1 square foot of
heated surface per hour, from 212¡.
8.76 lbs. of water evaporated by 1 lb. of coal from
212¡."
Locomotive Boiler.
"1 lb. of coke burnt under one boiler in 6.45
seconds.
79.33 lbs. of coke burnt on each square foot of grate,
per hour.
12 lbs. of water evaporated by 1 square foot of heated
surface per hour.
7.12 Ibs. of water evaporated by 1 lb. of coke from
212¡.
5.70 lbs. of water evaporated by 1 lb. of coal from
212¡, by calculation.
5.11 lbs. of water evaporated by 1 lb. of coal from
212¡, by Wood's experiment."
Go to the Anthracite Iron Page
About The Hopkin Thomas Project
Rev. June 2010